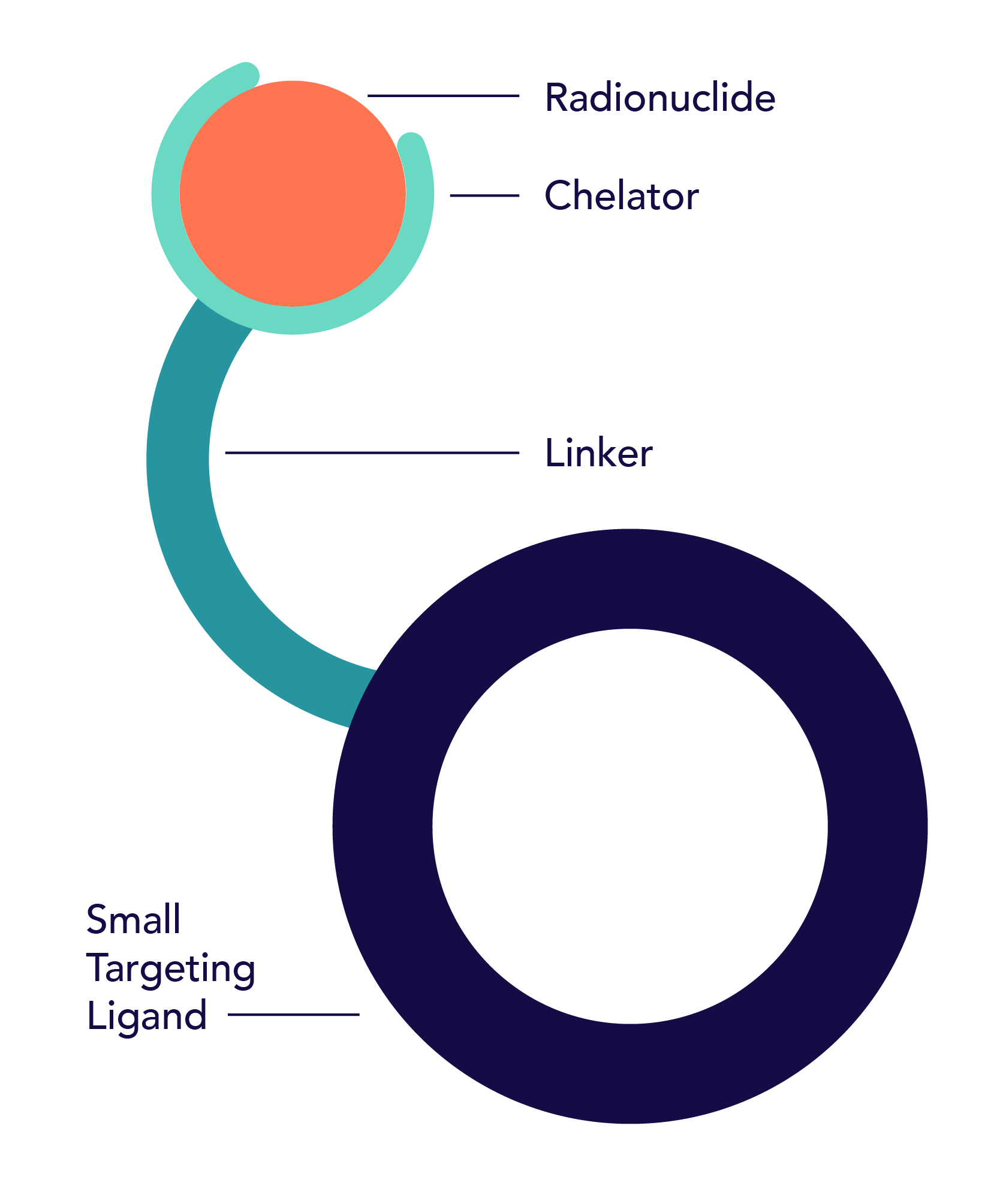
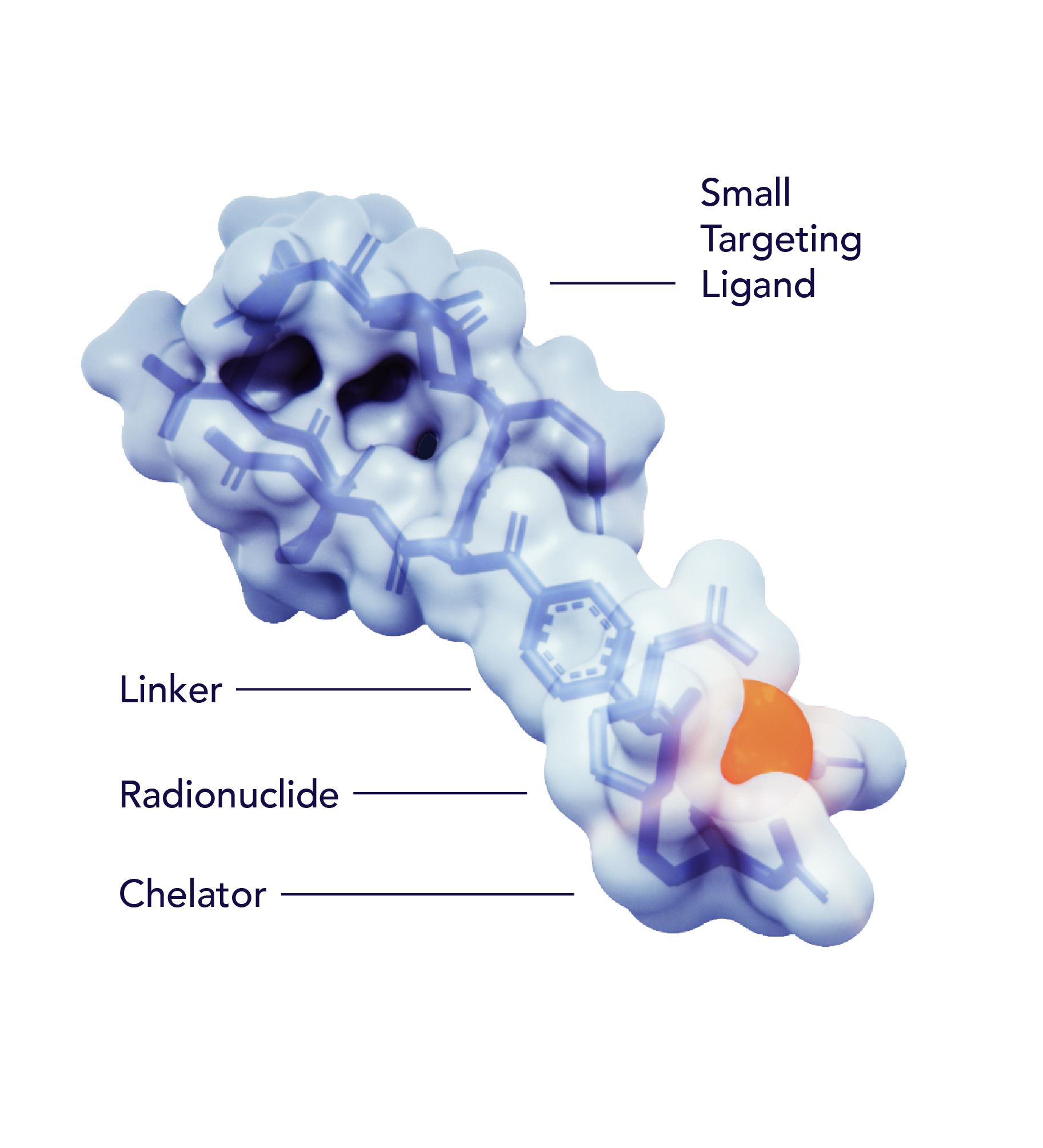
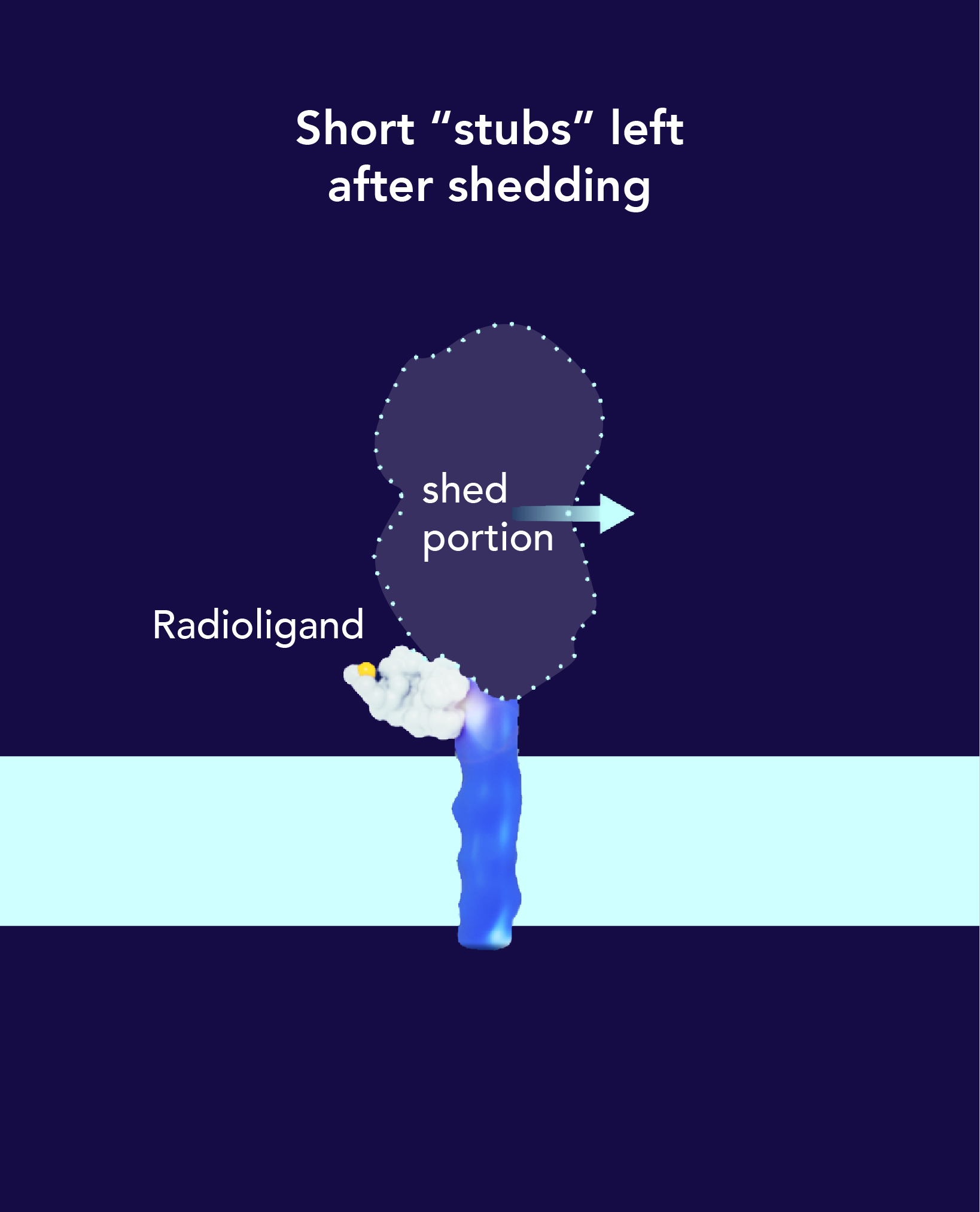
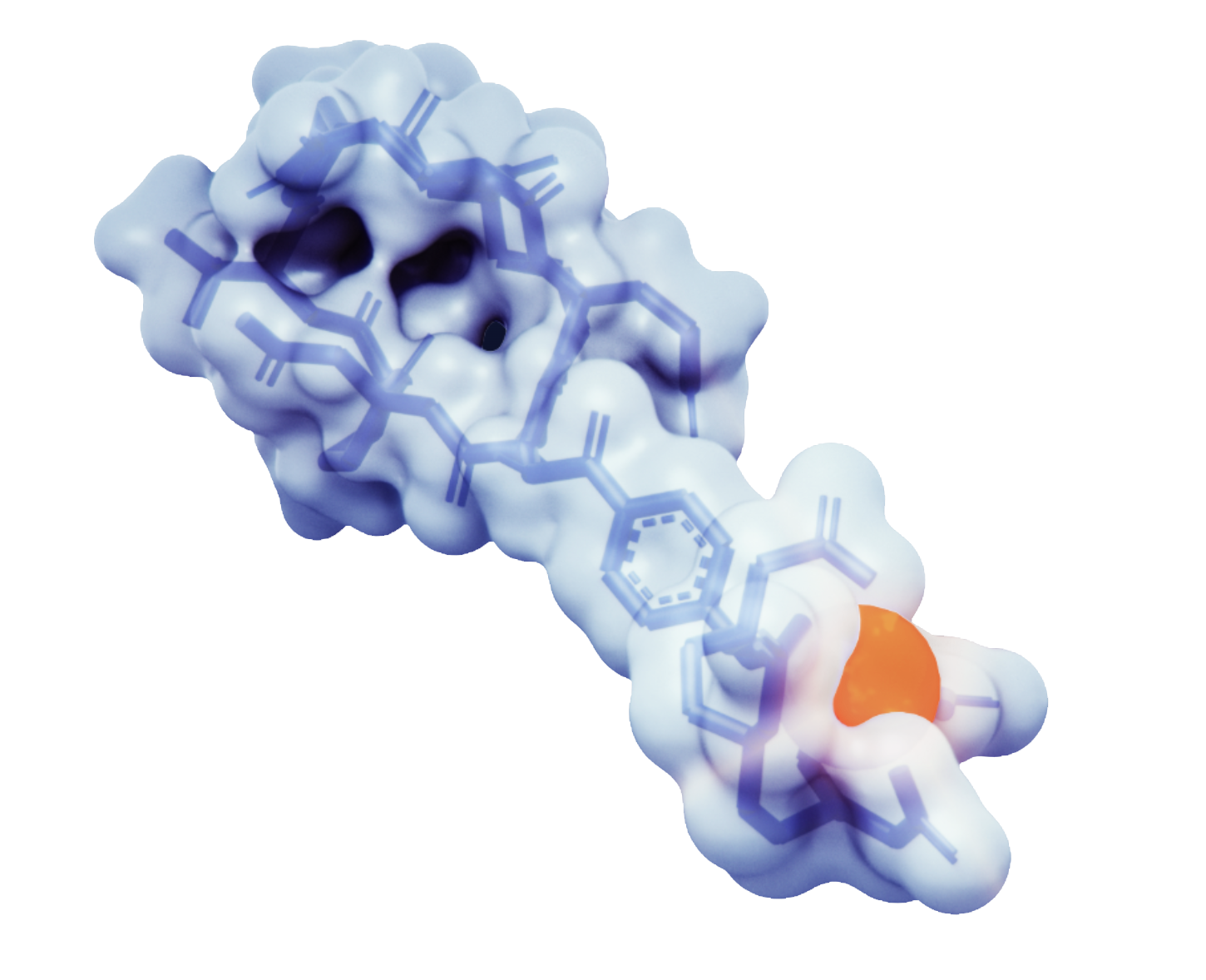
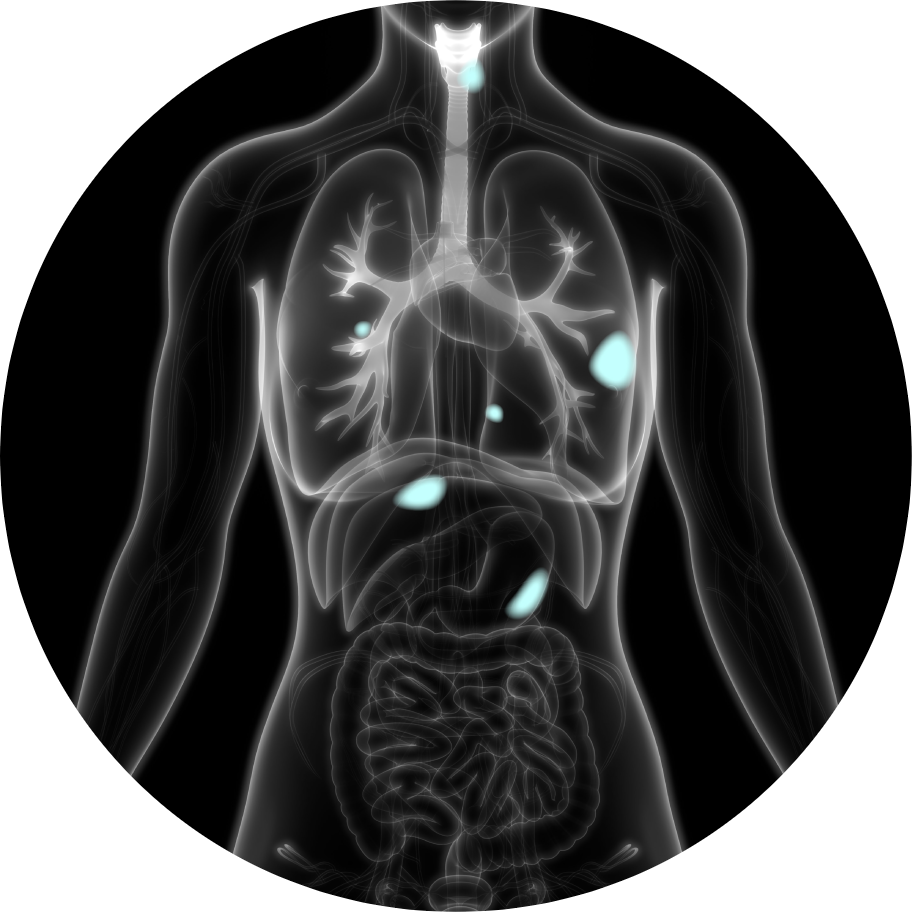
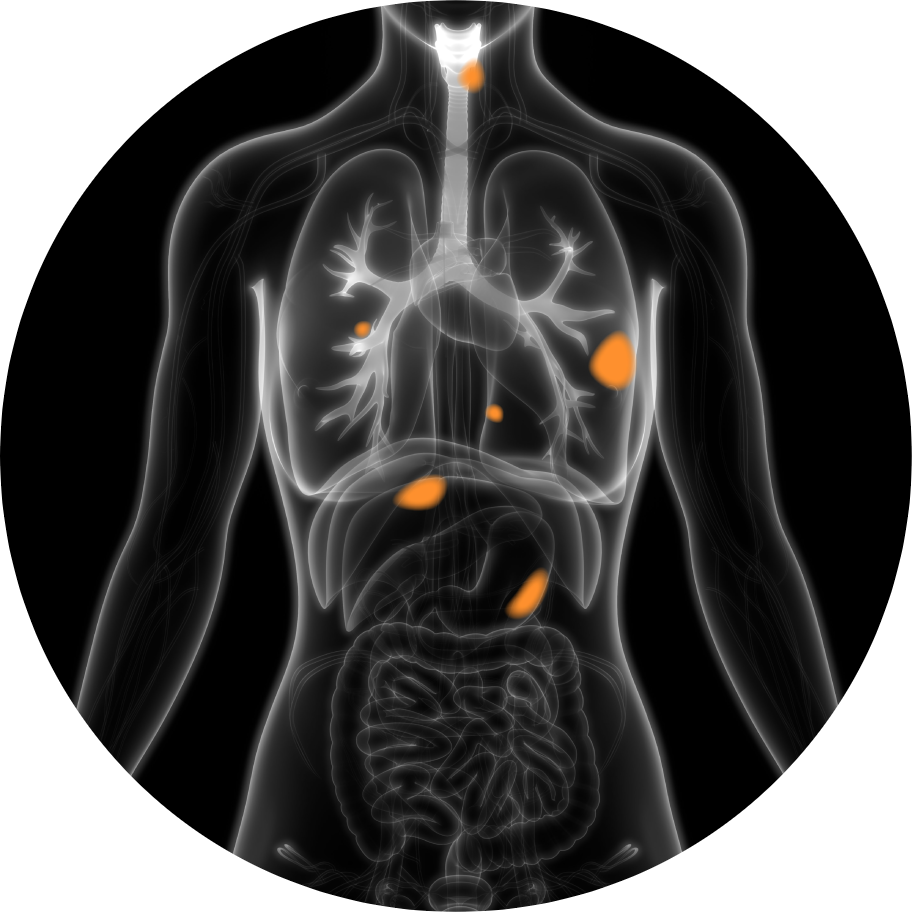
A radiopharmaceutical is an agent designed to selectively identify and deliver radiation to the tumor site.
Radiopharmaceuticals are composed of three parts: a radioisotope that is protected by a chelator, a targeting ligand, and a linker that binds the radioisotope and ligand together.
The radioisotope can be a therapeutic high energy cell-killing one, like Actinium or Lutetium, or a lower energy imaging one, like Gallium, that does not damage cells.
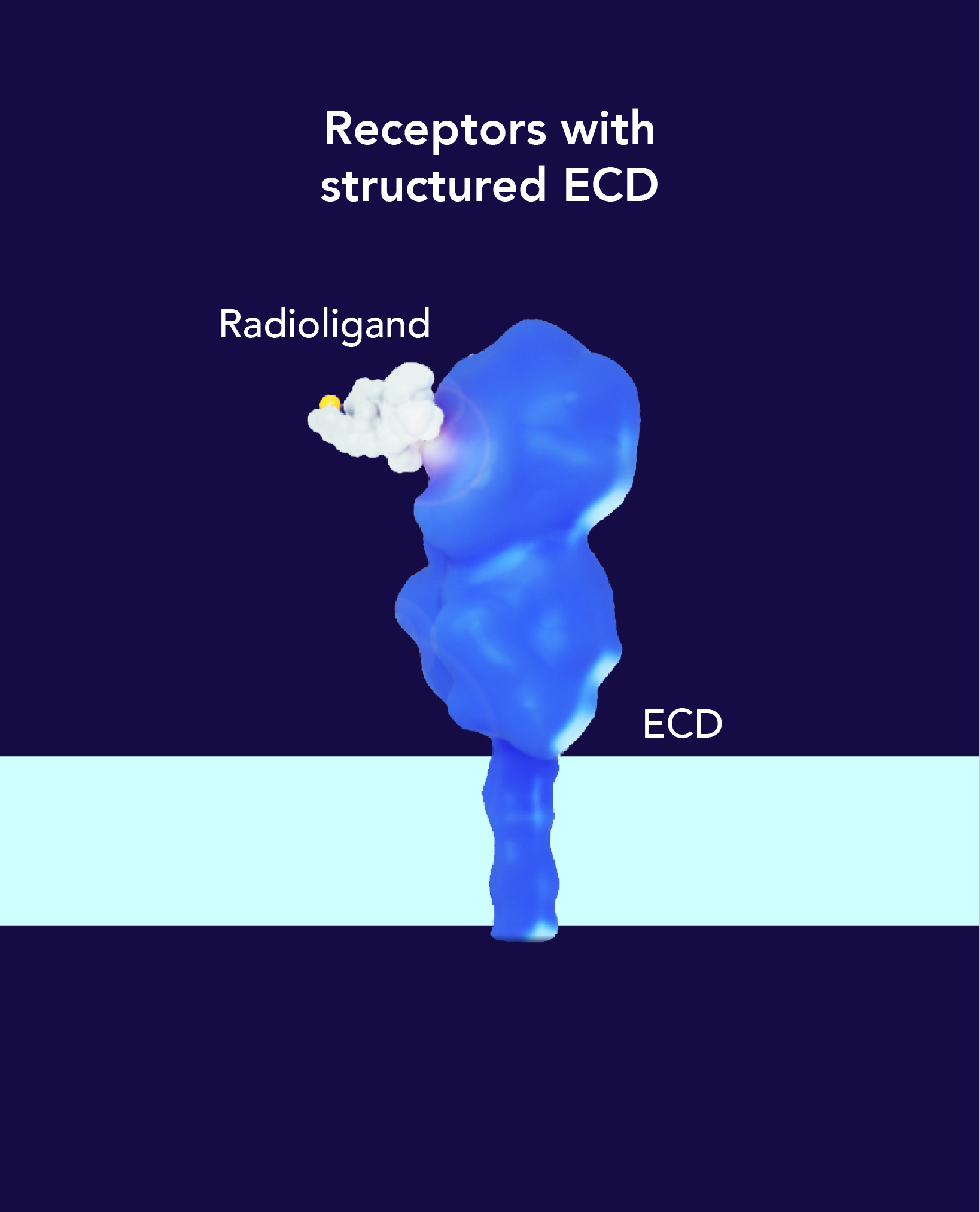
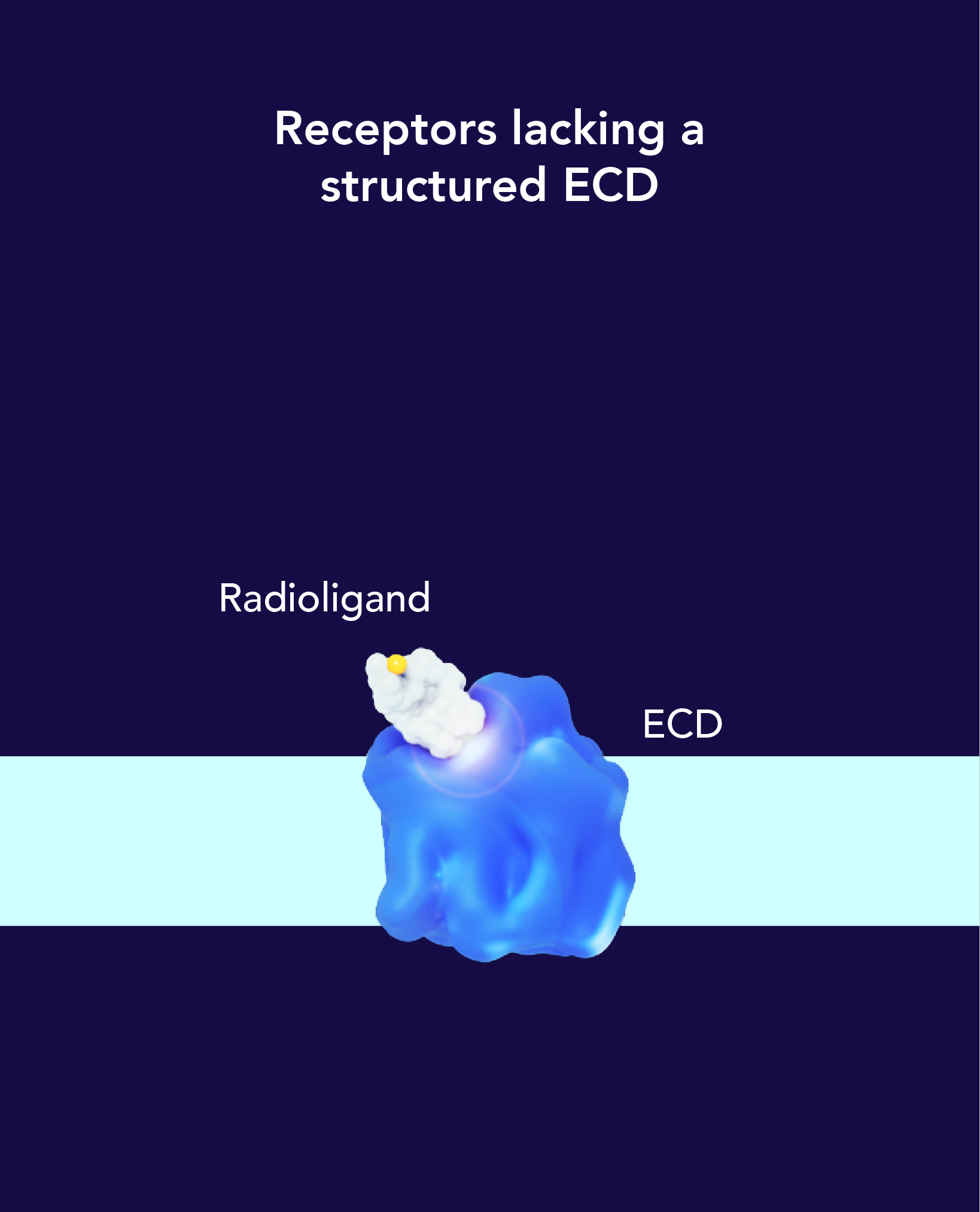
The targeting ligand delivers the radioisotope directly to a specific organ, tissue or cell that expresses the target, while avoiding normal tissues that do not express its target.